

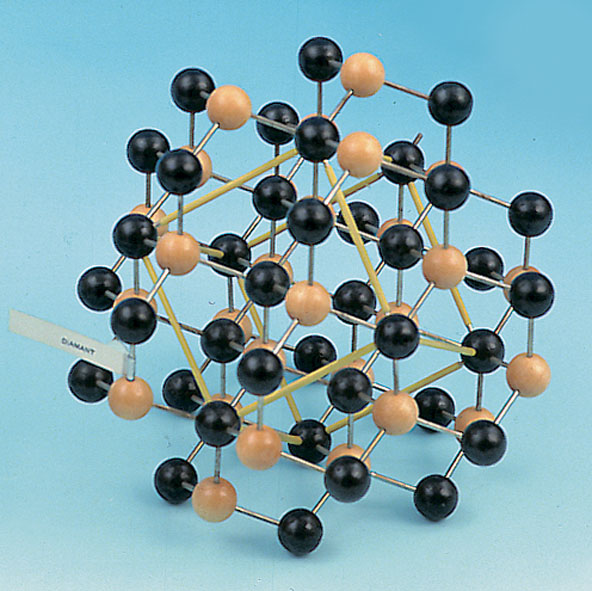
Solid crystals have both short-range order and long-range order. Certain liquids may have short-range order in one direction and long-range order in another direction these special substances are called liquid crystals. These fluids, such as water, have short-range order but lack long-range order. At distances that are many atoms away, however, the positions of the atoms become uncorrelated. In many liquids the first-neighbour atoms are arranged in the same structure as in the corresponding solid phase. Short range is defined as the first- or second-nearest neighbours of an atom. Most liquids lack long-range order, although many have short-range order. Once the positions of an atom and its neighbours are known at one point, the place of each atom is known precisely throughout the crystal. Long- and short-range orderĪ solid is crystalline if it has long-range order. This strong bond is responsible for making diamond the hardest solid. The covalent bond between two carbon (C) atoms is the strongest bond found in nature. The iron (Fe) atom, in contrast, has one electron shell that is only partially filled, giving the atom a net magnetic moment. In solid argon the atoms are arranged according to the closest packing of these spheres. The properties of solids are usually predictable from the properties of their constituent atoms and molecules, and the different shell structures of atoms are therefore responsible for the diversity of solids.Īll occupied shells of the argon (Ar) atom, for example, are filled, resulting in a spherical atomic shape. The arrangement of an atom’s electrons determines its chemical properties. Different atoms have different numbers of electrons, which are distributed in a characteristic electronic structure of filled and partially filled shells. The shells are filled in a systematic order, with each shell accommodating only a small number of electrons. The electrons of an atom move in orbits that form a shell structure around the nucleus. The basic units of solids are either atoms or atoms that have combined into molecules. A solid must keep its shape longer than that.ĭo you get fired up about physics? Giddy about geology? Sort out science fact from fiction with these questions. A highly viscous fluid retains its shape for an hour but not a year. The pertinent issue is how long the object keeps its shape.
A possible definition of a solid is an object that retains its shape if left undisturbed. They are called solids at all temperatures below their melting point.
Many crystals behave like butter in that they are hard at low temperatures but soft at higher temperatures. After remaining on the kitchen counter for a day, the same cube becomes quite soft, and it is unclear if the butter should still be considered a solid. A cube of butter, for example, is hard after being stored in a refrigerator and is clearly a solid. Upon inspection, however, the definition becomes less straightforward. The definition of a solid appears obvious a solid is generally thought of as being hard and firm. See all videos for this articleĬrystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Created and produced by QA International. Minerals crystallize according to one of seven motifs, known as crystal systems.

CRYSTAL LATTICE HOW TO


 0 kommentar(er)
0 kommentar(er)
